Hey there! Ready to dive into the fascinating world of chemistry and learn about double replacement reactions? Well, you’re in luck! Today, we’ll explore the ins and outs of this type of reaction and discover how it works on a molecular level. So, what exactly is a double replacement reaction in chemistry?
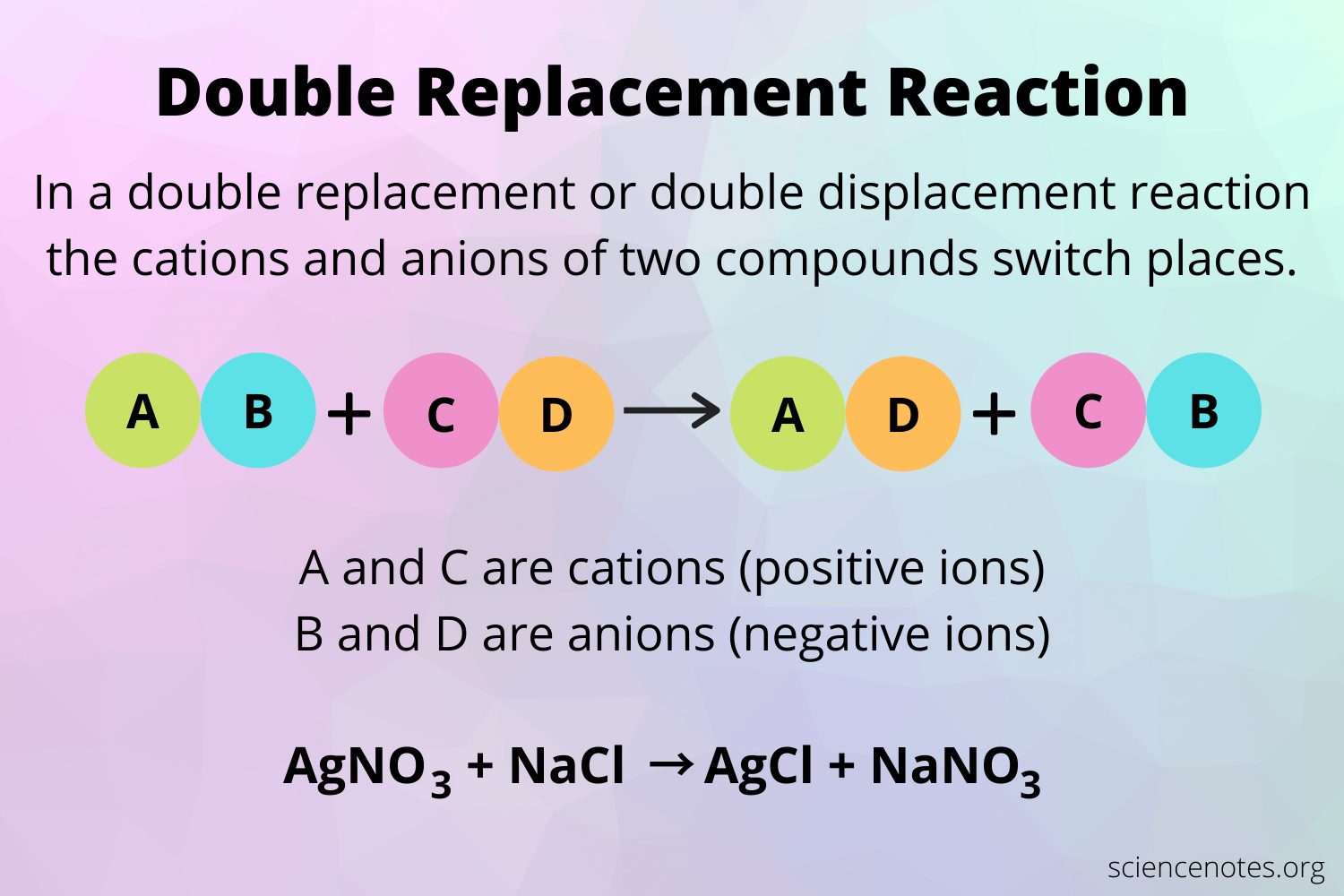
In simple terms, a double replacement reaction occurs when two compounds swap partners to form two new compounds. It’s like a chemical dance party where the partners switch and create something entirely different! These reactions often take place in solutions and are commonly seen in various chemical processes.
But why should we care about double replacement reactions? Well, they play a crucial role in everyday life. From the food we eat to the medicines we take, double replacement reactions are behind many chemical reactions that occur in our bodies and the world around us. So, let’s buckle up and explore the exciting world of double replacement reactions together!
What Is a Double Replacement Reaction in Chemistry?
A double replacement reaction, also known as a double displacement reaction or metathesis reaction, is a type of chemical reaction that occurs when two compounds exchange ions or elements with each other to form new compounds. This reaction takes place in aqueous solutions or when two ionic compounds are mixed together.
In a double replacement reaction, the positive and negative ions of two compounds switch places, resulting in the formation of two new compounds. The reactants and products in this reaction are usually written in the form of chemical equations, where the compounds are represented by their chemical formulas.
1. How Does a Double Replacement Reaction Work?
A double replacement reaction occurs when ions or elements in two compounds switch places with each other. This exchange of ions or elements happens as a result of the formation of new compounds that are more stable or that have a lower energy state.
For example, in the reaction between silver nitrate (AgNO3) and sodium chloride (NaCl), the silver cation (Ag+) from silver nitrate combines with the chloride anion (Cl-) from sodium chloride to form silver chloride (AgCl). At the same time, the sodium cation (Na+) from sodium chloride combines with the nitrate anion (NO3-) from silver nitrate to form sodium nitrate (NaNO3).
The chemical equation for this reaction can be written as:
AgNO3 + NaCl → AgCl + NaNO3
Here, the positive ions (Ag+ and Na+) and the negative ions (NO3- and Cl-) switch places to form the new compounds on the right side of the equation.
2. Examples of Double Replacement Reactions
Double replacement reactions are common in chemistry and have various applications in industries, laboratories, and everyday life. Here are some examples of double replacement reactions:
- Neutralization Reactions: When an acid reacts with a base, a double replacement reaction occurs. The hydrogen cation (H+) from the acid combines with the hydroxide anion (OH-) from the base to form water (H2O), while the positive and negative ions from the acid and the base combine to form a salt.
- Precipitation Reactions: When two aqueous solutions containing different ions are mixed, a double replacement reaction can result in the formation of an insoluble compound called a precipitate. This reaction is often used to determine the presence of specific ions in a solution.
- Metathesis Reactions: Metathesis reactions involve the exchange of ligands or ions between two compounds. These reactions are commonly observed in inorganic chemistry and can result in the formation of new compounds with different physical and chemical properties.
- Complex Formation Reactions: In complex formation reactions, two compounds react to form a complex compound in which one of the reactants acts as a ligand, surrounding a central metal ion or atom.
3. Factors Affecting Double Replacement Reactions
Several factors can influence the rate and outcome of a double replacement reaction:
- Concentration: Higher concentrations of reactants can increase the likelihood of successful collisions between ions, leading to a faster reaction.
- Temperature: An increase in temperature generally speeds up a chemical reaction, as it provides more kinetic energy to the particles, allowing them to move faster and collide more frequently.
- pH: The pH of the solution can affect the reaction rate and the solubility of the resulting compounds.
- Precipitate Formation: In some double replacement reactions, the formation of a precipitate can influence the reaction by removing ions from the solution and shifting the equilibrium.
- Solvent: The nature of the solvent, whether it is polar or nonpolar, can impact the solubility of the compounds and therefore affect the reaction.
4. Applications of Double Replacement Reactions
Double replacement reactions have important applications in various fields:
- Pharmaceuticals: Double replacement reactions are commonly used in the synthesis of pharmaceutical drugs.
- Water Treatment: Precipitation reactions resulting from double replacement reactions are used in water treatment to remove contaminants.
- Metallurgy: Double replacement reactions are involved in the extraction of metals from their ores.
- Analytical Chemistry: Precipitation reactions are widely used in analytical chemistry for the determination of specific ions.
- Chemical Synthesis: Double replacement reactions are used to synthesize a wide range of chemical compounds.
5. Limitations and Challenges of Double Replacement Reactions
Despite their usefulness, double replacement reactions may encounter limitations and challenges:
- Competing Reactions: Sometimes, multiple different reactions can occur simultaneously, leading to side products or incomplete reactions.
- Solubility: The solubility of the resulting compounds plays a crucial role in determining whether a double replacement reaction will occur.
- Reactivity: The reactivity of the compounds involved in the reaction can affect the reaction rate and the success of the reaction.
- pH Sensitivity: Some double replacement reactions may require specific pH conditions to proceed efficiently.
6. Safety Considerations in Double Replacement Reactions
When conducting double replacement reactions, it is essential to observe safety precautions:
- Wear appropriate personal protective equipment, such as gloves and safety goggles.
- Work in a well-ventilated area or under a fume hood to prevent the inhalation of harmful gases.
- Handle chemicals carefully and avoid skin contact or ingestion.
- Follow proper disposal procedures for chemical waste.
7. Conclusion
Double replacement reactions are fascinating chemical processes in which two compounds exchange ions or elements, resulting in the formation of new compounds. These reactions have various applications in industries, laboratories, and everyday life. By understanding the principles and factors that influence double replacement reactions, scientists and researchers can harness their potential to synthesize new compounds, analyze solutions, and solve complex chemical problems.
Key Takeaways: What Is a Double Replacement Reaction in Chemistry?
- A double replacement reaction is a chemical reaction where two compounds exchange positive ions to form new compounds.
- These reactions are also known as metathesis reactions.
- Double replacement reactions often occur in aqueous solutions.
- There are several types of double replacement reactions, including precipitation reactions, acid-base reactions, and gas formation reactions.
- The products of a double replacement reaction can be predicted using the concept of solubility rules.
Frequently Asked Questions
Welcome to our guide on double replacement reactions in chemistry! Below, you’ll find answers to common questions surrounding this topic. Whether you’re a student or just curious about chemical reactions, we’ve got you covered.
Q: What is a double replacement reaction in chemistry?
A double replacement reaction, also known as a metathesis reaction, is a chemical reaction where the cations and anions of two different compounds switch places. This reaction typically occurs between two ionic compounds dissolved in a solution. The result is the formation of two new compounds, with swapped cations or anions.
For example, in a typical double replacement reaction, if two ionic compounds, AB and CD, are mixed together, AD and CB will be formed. The reaction can be represented using a chemical equation, such as AB + CD → AD + CB.
Q: What are some examples of double replacement reactions?
There are numerous examples of double replacement reactions that occur in everyday life and in laboratory experiments. One common example is the reaction between sodium chloride (NaCl) and silver nitrate (AgNO3). When these two compounds are mixed, a double replacement reaction occurs, resulting in the formation of sodium nitrate (NaNO3) and silver chloride (AgCl). The chemical equation for this reaction is NaCl + AgNO3 → NaNO3 + AgCl.
Another example is the reaction between barium chloride (BaCl2) and sulfuric acid (H2SO4). This reaction yields barium sulfate (BaSO4) and hydrochloric acid (HCl). The chemical equation for this reaction is BaCl2 + H2SO4 → BaSO4 + 2HCl.
Q: What determines if a double replacement reaction will occur?
To determine if a double replacement reaction will occur, several factors need to be considered. One of the key factors is the solubility of the compounds involved. If one of the products of the reaction is insoluble, a precipitate will form, indicating that a double replacement reaction has occurred.
Another factor that determines whether a double replacement reaction will occur is the exchange of ions. The ions present in the reactants must be able to trade places and form new compounds. If the exchange of ions does not result in the formation of stable compounds, the reaction might not take place.
Q: What are the applications of double replacement reactions?
Double replacement reactions have various applications in chemistry and other fields. One of their practical uses is in the production of useful compounds. For example, these reactions are often employed in the synthesis of pharmaceutical drugs, where specific compounds need to be created by swapping ions between reactants.
Double replacement reactions also play a role in analyzing the presence of certain ions in a solution. By observing the formation of precipitates and identifying the compounds produced, scientists and analysts can determine the composition of samples.
Q: How can double replacement reactions be balanced?
Balancing a double replacement reaction involves ensuring that the number of atoms of each element is the same on both sides of the chemical equation. This is achieved by adjusting the coefficients in front of each compound or molecule.
The process of balancing involves starting with elements that appear in only one compound, then progressing to elements that appear in multiple compounds. By manipulating coefficients and adding coefficients as needed, the reaction can be balanced. It’s important to note that the subscripts within compounds should never be changed when balancing a chemical equation.
Summary
So, we’ve learned a lot about double replacement reactions in chemistry. These reactions happen when two compounds swap their ions to form new compounds. Remember, compounds are just different elements joined together. When two compounds mix, the positive ions from one compound join with the negative ions from the other. It’s like a little dance where everyone switches partners!
In a double replacement reaction, these partner switches result in two new compounds being made. We talked about how to identify these reactions using a special notation called a balanced chemical equation. This equation shows the starting compounds and the new compounds that are formed. Double replacement reactions can have many different outcomes, like forming a solid, a gas, or even water! They happen all around us, from the chemicals in our food to the reactions that make different products. Understanding double replacement reactions is a key part of understanding how chemistry works in our everyday lives. So keep exploring and discovering more about the fascinating world of chemistry!
